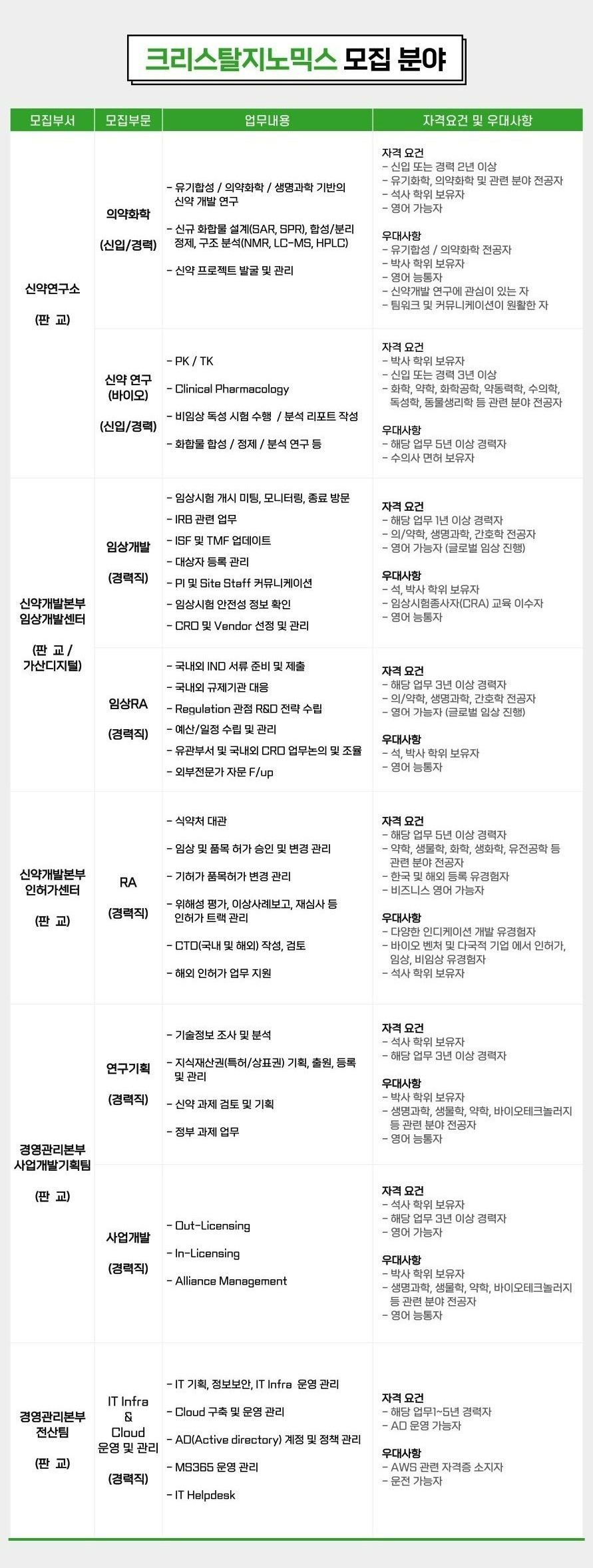
[CrystalGenomics] 2022 수시채용 (Pangyo, Korea)
크리스탈지노믹스㈜에서 KAPAL의 우수한 인재를 모십니다.
인류의 삶의 질(Quality of Life) 개선에 의의를 두고, 함께 세계적인 신약 연구∙개발의 꿈을 실현할 도전적이고 열정적인 인재를 찾습니다.
< 크리스탈지노믹스㈜ 소개 >
당사는 차세대 관절염 치료제, 내성 균주를 박멸하는 신개념 항생제, 분자 표적 항암제와 같이 환자수는 증가하지만 우수한 치료제가 없어 치료에 대한 불만족도가 높은 분야의 혁신 신약 개발에 집중하고 있습니다.
그리하여 질환 표적 단백질의 3차 구조 규명 기술을 포함하는 신약연구개발기술 시스템을 갖추고 신약 개발 파이프라인을 강화하기 위해 최선을 다하고 있습니다.
< 크리스탈지노믹스㈜ 주요 성과 >
– 국내 산학연 최초 신약발굴 기반기술을 활용하여 Nature 표지 논문 게재 (2003)
– 정부의 기술성평가제도 제1호로 코스닥에 상장 (2006)
– 국내 바이오벤처 최초로 혁신 신약 제22호 아셀렉스 승인, 시판 및 수출 (2015)
– 세계적 경쟁력의 신약 발굴 기반 기술로 지속적인 개발후보 발굴
CrystalGenomics, Inc. is headquartered in Pangyo, South Korea and has a U.S. subsidiary (CG Pharmaceuticals, Inc.) in Orinda California for the management of global clinical trials and is publicly traded on the KOSDAQ exchange.
CrystalGenomics is a research-oriented biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of highly unmet medical need.
CrystalGenomics is mainly focused in the therapeutic areas of cancer, infectious disease, pain and inflammation.
For more information, please visit: www.cgxinc.com or www.crystalgenomics.com
CrystalGenomics seeks for the candidates with the traits of integrity, teamwork, excellence, commitment and accountability.



[MEDIPOST] Team Leader / Team Member – Global Regulatory Affairs (Pangyo, Korea )
We are looking for a self-motivated individual to join our regulatory affairs department to provide regulatory support for international clinical development of innovative cell therapies. This individual will have direct support from leadership and team members through training and continuous learning.
Location: MEDIPOST HQ located in Pangyo, Gyeonggi-do, South Korea (On-site)
Open Positions: Team Leader, Team Member(s)
Primary Responsibilities
- Regulatory affairs duties related to clinical development and marketing authorization of cell and gene therapies in the international market.
- Prepare and review document package (CTD) and other regulatory deliverables for international regulatory submissions
- Interaction with the applicable regulatory authorities for assigned projects
- Manage overseas (Japan) clinical trials – timeline/CRO/CMO/ partners management
- Support clinical development activities of foreign subsidiary companies
Basic Qualification
- Bachelor’s degree and 5+ years of experience in biology, life sciences or health-related field.
- Excellent verbal and written English and Korean communication skill
- Strong interpersonal, teamwork and organizational skills
Preferred Qualification
- Advanced degree (Master) in biology, life science, health-related field or regulatory affairs
- Experience in regulatory submissions for Advanced Therapy Medical Products (ATMPs) / Biologics
- GMP QA experience
- CRA experience
- Japanese language skill
Contact: Sophia Yang – sophia.yang@medipostamerica.com
About MEDIPOST
MEDIPOST is a Korean company founded in 2000 and became a public company in 2005, listed on KOSDAQ with 300+ current employees. MEDIPOST operates 2 overseas subsidiary companies in Tokyo, Japan and Gaithersburg MD, U.S.
MEDIPOST operates the largest private cord blood bank “CELLTREE®” in Korea with over 255,000 units of private cord blood units under storage. Each year, over 20,000 private cord blood units are collected and stored at CELLTREE®.
MEDIPOST’s research and development is focused on novel off-the-shelf, allogeneic cell therapeutics using human Umbilical Cord Blood-derived Mesenchymal Stromal Cells (hUCB-MSCs) with clinical-stage assets in the disease areas of osteoarthritis (OA), broncho-pulmonary dysplasia (BPD) and Alzheimer’s disease (AD).
MEDIPOST’s flagship product, CARTISTEM® (allogeneic Umbilical Cord Blood-derived MSCs + hyaluronic acid hydrogel composite) for knee osteoarthritis was approved in 2012 by Korea’s regulatory agency Ministry of Food and Drug Safety (MFDS) with Biologics License Application (BLA) label “Treatment of knee articular cartilage defects in patients with osteoarthritis (ICRS grade IV) as a result of degenerative disease or repeated trauma (without age limit)”. To date, over 21,000 patients have been treated on the market with an excellent long-term safety and efficacy profile. CARTISTEM® has also successfully completed the Phase I/II trial in the U.S. and the Phase III trial is planned to commence in 2023. CARTISTEM® is currently undergoing Phase III clinical trial in Japan, while BLA marketing-authorization is under review in Malaysia.
PNEUMOSTEM® (allogeneic Umbilical Cord Blood-derived MSCs) for the prevention of Bronchopulmonary Dysplasia (BPD) in premature infants, completed the first-in-human Phase I safety trial in Korea and the randomized, placebo-controlled Phase II clinical trial is ongoing in Korea. Phase I/II clinical trial in the U.S. has been completed confirming safety with positive efficacy signals. Longer term (3, 4 and 5 year) follow-up on premature infants born with high-risk of developing BPD who received PNEUMOSTEM® demonstrated significant benefit with regards to cognitive development compared to cohort of premature infants who received standard-care alone. PNEUMOSTEM® has received Orphan Drug Designation (ODD) and the Fast-Track Designation (FTD) by the US-FDA and ODD by the EMA.
MEDIPOST’s 2nd generation human Umbilical Cord Blood-derived Mesenchymal Stromal Cell (hUCB-MSC) pipeline code named SMUP-IA-01 is an off-the-shelf intra-articular injectable cell product for the patients with early to mid-stage knee osteoarthritis (OA). SMUP-IA-01 has completed dosing of all 12 subjects in the first-in-human Phase 1 clinical trial in Korea and the 12-months follow-up data demonstrated significant and sustained improvement in subjective pain and knee function assessments in all subjects, with longer-term follow-up planned. Phase 2 randomized controlled trial in Korea is currently ongoing.
SMUP platform is MEDIPOST’s proprietary, patent-protected cell selection and expansion technology using allogeneic umbilical cord blood-derived stem cells as a therapeutic modality. We are looking for qualified and experienced scientists to embark on the exciting journey of developing innovative cell and gene-modified cell therapy programs for unmet medical needs at MEDIPOST.
KOSDAQ ticker: 078160
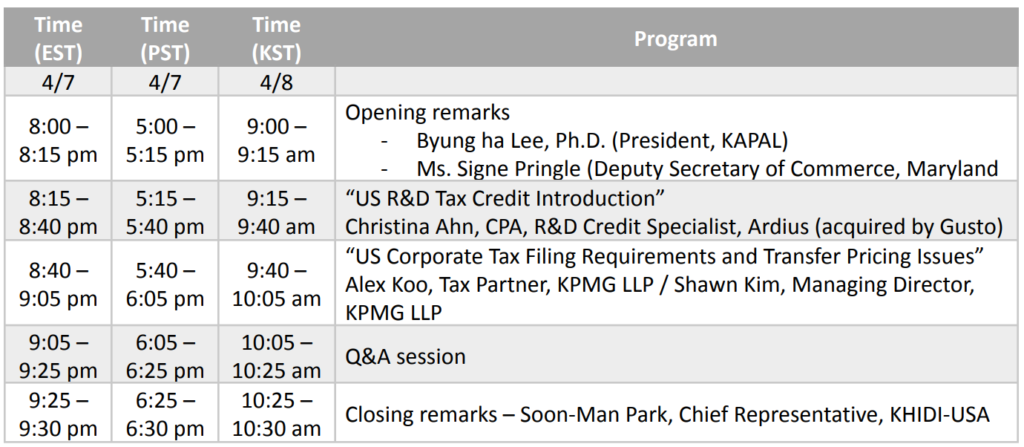
10th KAPAL On-Air Webinar (2022 KAPAL-KHIDI Webinar Series #1) (April 7, 2021, 8:00 – 9:30 PM EST)
2022 KAPAL–KHIDI Bio & Health Webinar Series는 한미생명과학인 협회 (KAPAL)와 KHIDI 미국지사 웨비나 시리즈입니다.
Topic Title: US R&D Tax Credit & Corporate Tax
o “US R&D Tax Credit Introduction”
Christina Ahn, CPA, R&D Credit Specialist, Ardius (acquired by Gusto)
o “US Corporate Tax Filing Requirements and Transfer Pricing Issues”
Alex Koo, Tax Partner, KPMG LLP / Shawn Kim, Managing Director, KPMG LLP

About the presenter:
Christina Ahn, CPA
R&D Credit Specialist, Ardius (acquired by Gusto)
Co-founder and CEO, Pinecone41
Pinecone41 is a Fintech startup that specializes in R&D Tax Credit services and provide technology startups accurate and beneficial tax insights that can be used to fuel growth.
Top Skills
Financial Analysis, Data Analysis, Corporate Tax
Experience
Ardius (acquired by Gusto, R&D Credit Specialist), Pinecone41 (CEO), KPMG (Tax Manager), Deloitte Tax LLP (Senior Tax Consultant), Mah & Associates (Staff Accountant)
Alex Koo
Tax Partner, KPMG LLP
Alex is a Tax Partner in KPMG’s Los Angeles Federal Tax Services, Korean practice. Before joining KPMG, Alex started his career in the tax department of Deloitte Korea and performed various tax projects until he was seconded to the US office. He has more than 20 years of experience in U.S. and Korea tax consulting, income tax compliance and income tax provision.
Alex is responsible for a broad range of U.S. multi-national and foreign-owned clients doing business as single entities or joint ventures, and has extensive experience with a wide range of tax matters relating to multi-national businesses. Alex’s current and past clients include leaders in the industrial production industries and high-technology, media, and telecommunication industries.
Alex has provided tax compliance services for various engagements in size from private to multi-national public companies in the U.S. and Korea and has extensive experience in defending corporations against tax examinations by Federal and state tax authorities.
Alex also has provided tax consulting services related to merger & acquisition transactions, business transfer transactions, cross border transactions and various tax due diligence services.
Alex has strong tax technical skills and tax provision skills with extensive experience in ASC 740 and ASC 718-10 for multinational and private companies.
Shawn Kim
Managing Director, KPMG LLP
Sanghoon has over 18 years of experience in dealing with audit, tax and transfer pricing matters for multinationals operating in different industries, from manufacturing and distribution sectors to the financial industry.
Sanghoon manages tax planning, audit defense and documentation projects for significant and complex intercompany transactions, covering tangible goods, complex services, intellectual property and financing structures. He deals with tax authorities on behalf of taxpayers with respect to transfer pricing audit defense and Advance Pricing Arrangements.
Work Experience
• Dealt with tax authorities related to tax audit, tax appeal, advance pricing agreement (APA), and mutual agreement procedure (MAP)
• Assisted companies in preparing worldwide transfer pricing policies
• Assisted companies in preparing FIN 48 analyses
• Prepared transfer pricing planning study and contemporaneous transfer pricing documentations
• Prepared management service fee documentation
• Performed intellectual property valuation analyses
• Performed royalty analysis products, especially in automobile parts industry
***** Notice *****
* Please note that the webinar talk will be presented in ENGLISH language. But Q&A session will be conducted in both English and Korean.
* 이번 세미나는 Maryland 주정부 관계자 분들을 포함하여 영어권 참석자 분들이 있어, 강의는 영어로 진행될 예정입니다. 참고로 질의 응답은 영어와 한국어 모두 가능함을 안내 드립니다.

Dr. Johng Sik Rhim Obityary (1930 – 2022) – Funeral service on Mar 10, 11 am @ Joseph Gawler’s Sons, LLC
https://www.legacy.com/us/obituaries/washingtonpost/name/johng-rhim-obituary?id=33551824

Peacefully passed away on March 5, 2022 in Bethesda, MD. Born July 24, 1930 in Gwangju, South Korea. A distinguished physician, virologist and cancer research scientist who worked with Dr. Albert Sabin on the polio vaccine, had numerous publications and scientific patents during a 45 year career at the National Institutes of Health and Uniformed Services University of the Health Sciences, and was a local business owner. Johng was the beloved husband of the late Mary Lytle Rhim; loving father of Jonathan Arch Rhim (Thanh), Christopher Huc Rhim (Lauren), Peter Kennedy Rhim (Jill), Andrew Lytle Rhim (Darshini), Michael Johng Rhim (Laura), and Kathleen Rhim Goodner (Ryan); devoted grandfather of Natalie and Aaron, Brendan and Abigail, Annie and Sarah and Rachel, Anika and Aidan, Nicolas and Claudia, and Emma and Cash. Relatives and friends are invited to call at Joseph Gawler’s Sons, LLC, 5130 Wisconsin Ave., NW, Washington, DC on Thursday, March 10, 2022 from 9 a.m. to 11 a.m., with funeral service to follow at 11 a.m. In lieu of flowers or other gifts, memorial contributions may be made in his name to the Foundation for the National Institutes of Health (F-NIH), www.FNIH.org.
Published by The Washington Post from Mar. 8 to Mar. 10, 2022.To plant trees in memory, please visit the Sympathy Store.
Virtual Funeral – Live Stream
Instead of flowers, we encourage people to donate in his name to the Foundation for the National Institutes of Health (F-NIH), www.FNIH.org.
Service – Thursday, March 10
Location – Joseph Gawler’s Sons, LLC (5130 Wisconsin Avenue, Washington, DC 20016)
Schedule for Thursday:
9:00am ~ 11:00am – viewing
11:00am ~ 12:00pm – service
12:00pm ~ 2:00pm – light fare/visitation at Gawler’s
5:00pm ~ 8:00pm – visitation at Rhim home (11455 South Glen Road, Potomac, MD 20854) Note:Parking will likely be a zoo – park on side of 11455 South Glen driveway or on parallel driveway 11401/11405/11409 South Glen


